Hydrogen sol
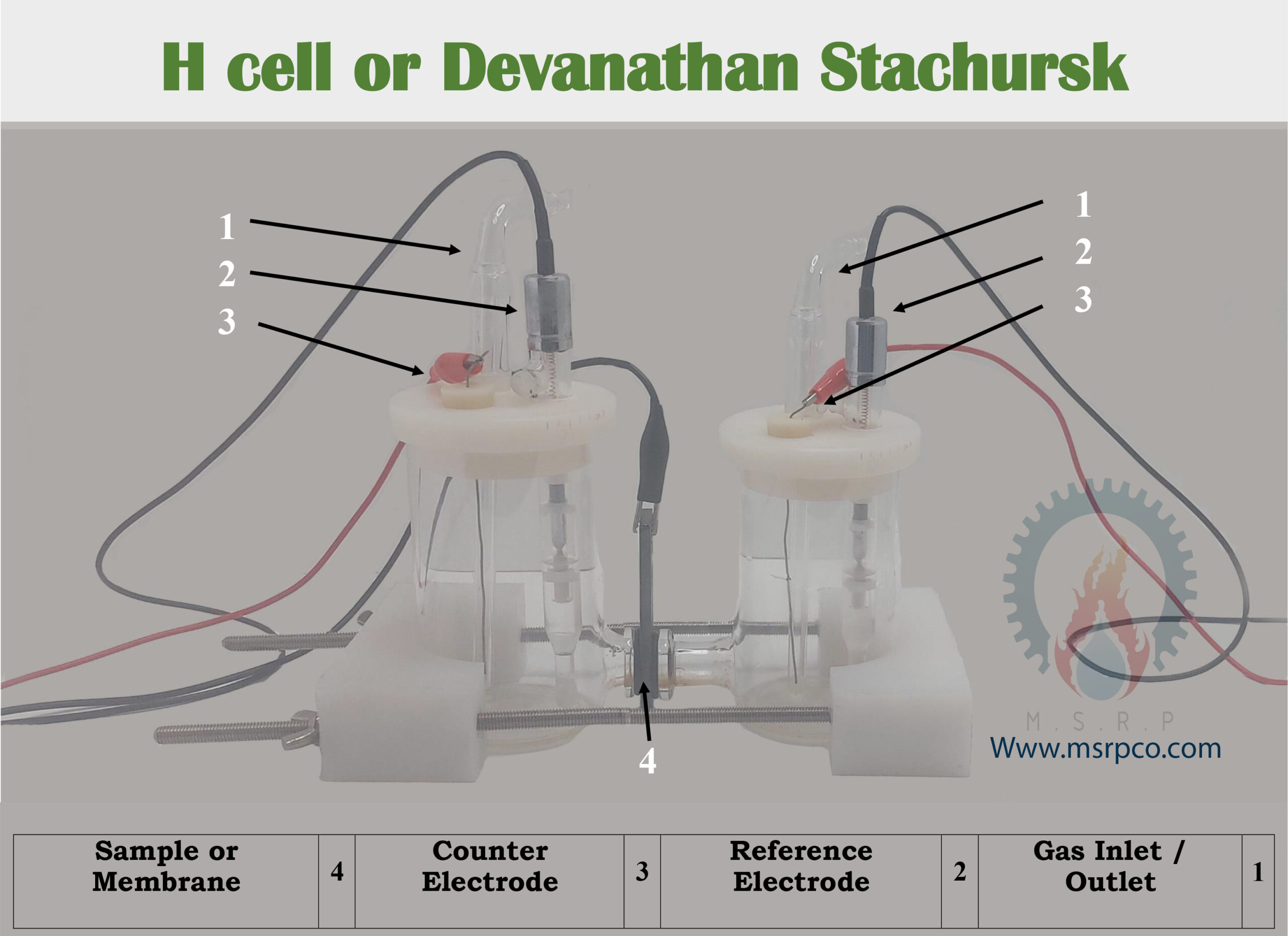
Hydrogen cell or Devanathan Stachursk is an electrochemical cell consisting of two cells with a volume of approximately 50 cc. According to the ASTM G148-97 standard the assembly consists of two separate chambers. Each cell, in addition to the possibility of gas injection, has the ability to place reference and counter electrodes simultaneously. Corrosion cells are used to check the penetration of hydrogen gas in steel and other alloys, as well as to check ion exchange membranes. In this method the current is applied on the polarized electrodes and the potential is measured separately in the reference electrodes.
To check and measure the penetration rate, both sides of the sample, which is a metal strip or an electrically conductive ion exchange membrane, are used as working electrodes for both cells. These tubes provide the possibility of investigating corrosion behavior in samples with two different compositions or hydrogen concentration. For this purpose, two simultaneous polarization or impedance tests are performed on the same sample in two different environments (in terms of solution composition or gas concentration). In order to investigate the behavior of concentration batteries in a chamber, the sample is charged potentiostatically or galvanostatically with hydrogen and the electrolyte circulates in cell. In the second chamber on the other side, the penetrant is anodically polarized. Therefore, the diffused hydrogen is completely oxidized and the amount of diffused hydrogen is calculated with the help of anode current.